Spatio-Temporal Quantification of FRET in living cells by fast time-domain FLIM: a comparative study of non-fitting methods
A. Leray, S. Padilla-Parra, J. Roul, L. Héliot and M Tramier
PLoS One 8, e69335 (2013) [Accès à la revue]
Le FRET est une autre technique de choix pour détecter des interactions entre protéines, mais il fallait plus d’une minute pour obtenir une image des temps de vie de fluorescence, et donc des complexes étudiés. Les techniques de FLIM (Fluorescence Life time Imaging Microscopy) rapide que nous avons développées permettent de réduire à une seconde ce temps d’acquisition et de limiter le stress oxydant lié à l’irradiation.
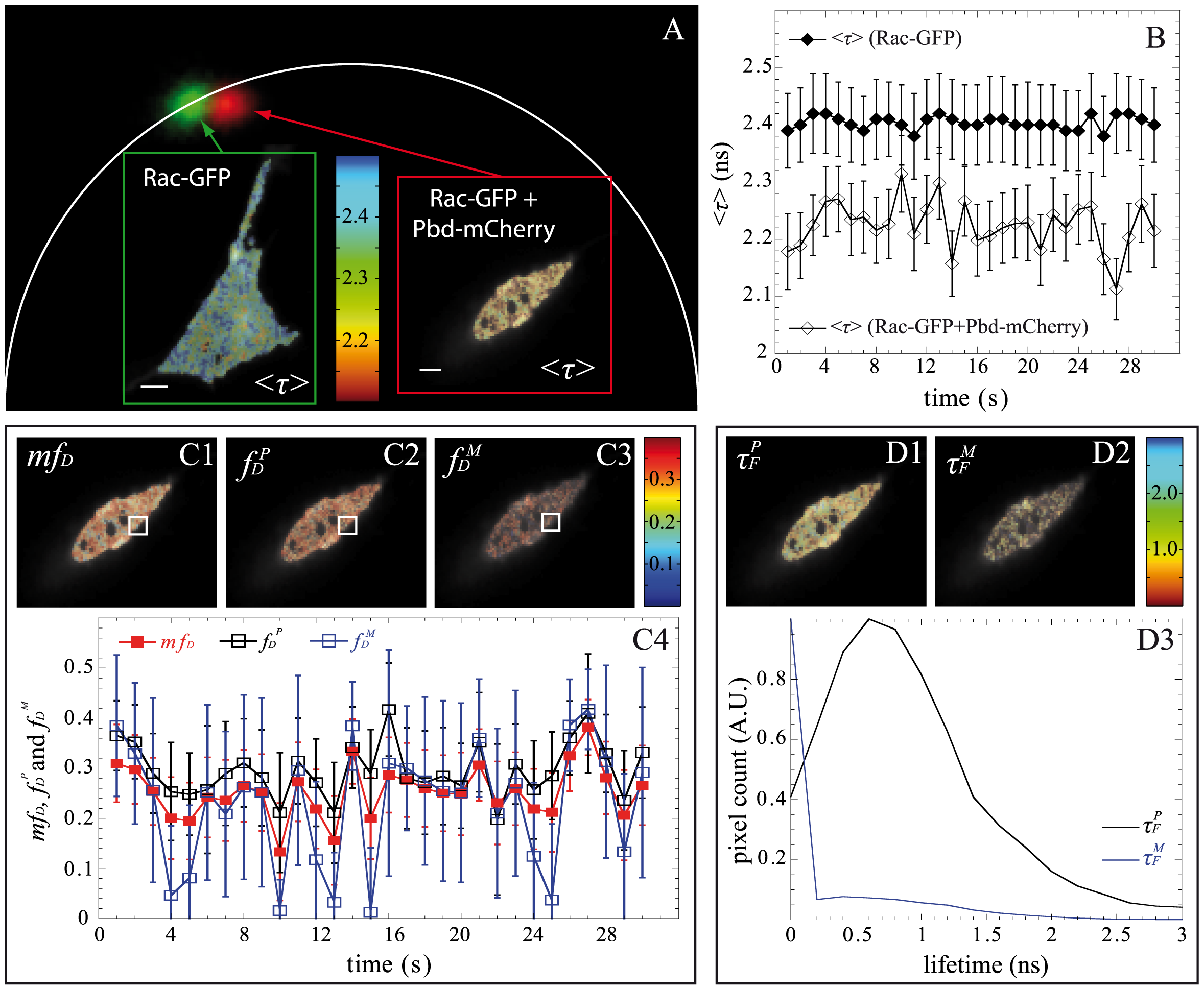
Abstract: Article About the Authors Metrics Comments Related Content Corrections (1) Abstract Introduction Materials and Methods Results Discussion Supporting Information Author Contributions References Reader Comments (0) Figures Corrections 2 Aug 2013: Leray A, Padilla-Parra S, Roul J, Héliot L, Tramier M (2013) Correction: 827Spatio-Temporal Quantification of FRET in Living Cells by Fast Time-Domain FLIM: A Comparative Study of Non-Fitting Methods. PLoS ONE 8(8): 10.1371/annotation/75f99136-45ec-4078-932e-07ed1242a73e. doi: 10.1371/annotation/75f99136-45ec-4078-932e-07ed1242a73e | View correction Abstract Förster Resonance Energy Transfer (FRET) measured with Fluorescence Lifetime Imaging Microscopy (FLIM) is a powerful technique to investigate spatio-temporal regulation of protein-protein interactions in living cells. When using standard fitting methods to analyze time domain FLIM, the correct estimation of the FRET parameters requires a high number of photons and therefore long acquisition times which are incompatible with the observation of dynamic protein-protein interactions. Recently, non-fitting strategies have been developed for the analysis of FLIM images: the polar plot or “phasor” and the minimal fraction of interacting donor mfD. We propose here a novel non-fitting strategy based on the calculation of moments. We then compare the performance of these three methods when shortening the acquisition time: either by reducing the number of counted photons N or the number of temporal channels Nch, which is particularly adapted for the original fast-FLIM prototype presented in this work that employs the time gated approach. Based on theoretical calculations, Monte Carlo simulations and experimental data, we determine the domain of validity of each method. We thus demonstrate that the polar approach remains accurate for a large range of conditions (low N, Nch or small fractions of interacting donor fD). The validity domain of the moments method is more restricted (not applicable when fD<0.25 or when Nch = 4) but it is more precise than the polar approach. We also demonstrate that the mfD is robust in all conditions and it is the most precise strategy; although it does not strictly provide the fraction of interacting donor. We show using the fast-FLIM prototype (with an acquisition rate up to 1 Hz) that these non-fitting strategies are very powerful for on-line analysis on a standard computer and thus for quantifying automatically the spatio-temporal activation of Rac-GTPase in living cells by FRET.
Thème : Biophotonique
