Tuning liver stiffness against tumours: an in vitro study using entrapped cells in tumour-like microcapsules.
Leal-Egaña A, Fritsch A, Heidebrecht F, Díaz-Cuenca A, Nowicki M, Bader A, Käs J.
J. Mech. Behav. Biomed. Mater. 9, 113-121 (2012) [Accès à la revue]
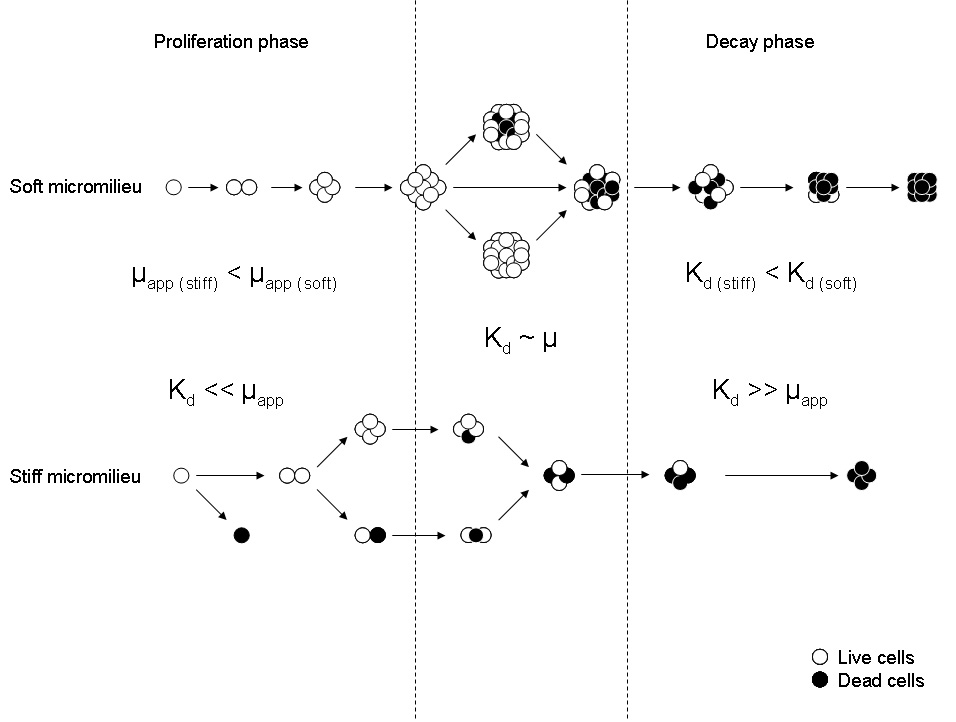
Abstract: Liver fibrosis is a reversible pathology characterized by the up-regulated secretion and deposition of ECM proteins and inhibitors of metalloproteinases, which increase the stiffness and viscosity of this organ. Since recent studies have shown that fibrosis preceded the generation of hepatocellular carcinomas, we hypothesize that liver fibrosis could play a role as a mechanism for restricting uncontrolled cell proliferation, inducing the mortality of cancer cells and subsequent development of primary tumours. With this purpose, in this work we analysed in vitro how the modulation of stiffness can influence proliferation, viability and aggregation of hepatocarcinoma cells (HepG(2)) embedded in 3D micromilieus mimicking values of elasticity of fibrotic liver tissues. Experiments were performed by immobilizing up to 10 HepG(2) cells within microcapsules made of 0.8%, 1.0% and 1.4% w/v alginate which, besides having values of elasticity from the lower-healthy to the upper-fibrotic range liver tissues, lacked domains for proteases, mimicking the micromilieu existing in hepatic primary tumours. Our results show that entrapped cells exhibited a short duplication phase followed by an irreversible decay stage, in which cell mortality could be mediated by two mechanisms: mechanical stress, in the case of cells entrapped in a stiffer micromilieu; and mass transfer limitations produced by pore coarsening at the interface cell-matrix, in softer micromilieus. According to the authors' knowledge, this work represents the first attempt to elucidate the role of liver fibrosis during Hepatocarcinoma pathologies, suggesting that the generation of a non-biodegradable and mechanically unfavourable environment surrounding cancer cells could control the proliferation, migration of metastatic cells and the subsequent development of primary tumours.
Thème : Thème 2011-2014 : Mécanique, adhésion et motilité
