Friction Mediates Scission of Tubular Membranes Scaffolded by BAR Proteins
M. Simunovic, J.-B. Manneville, H.-F. Renard, E. Evergren, K. Raghunathan, D. Bathia, A.K. Kenworthy, G.A. Voth, J. Prost, H. McMahon, L. Johannes, P. Bassereau and A. Callan-Jones.
Cell 170, 172-184 (2017) [Accès à la revue]
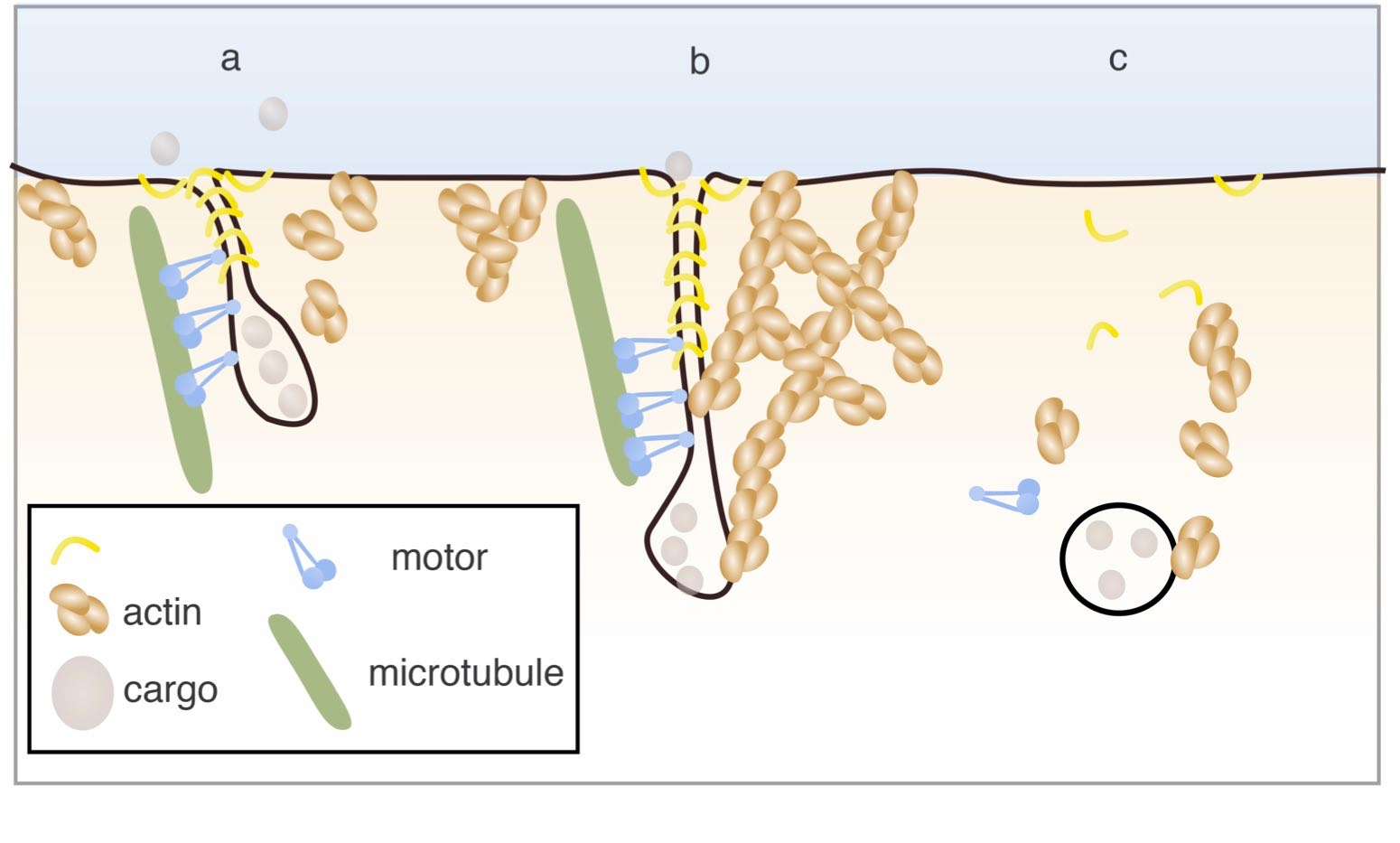
Membrane scission is essential for export of components between cellular compartments and with the exterior. Since cell membranes are fluid, specific "cutting" processes are required. We have shown that the assembly of a protein scaffold at the base of the vesicle or tubule (the transport system) can produce a friction with the membrane; then, when molecular motors pull on the tubule and extend it, the membrane of the tubule stretches until it ruptures, leading to the detachment of the tubule of the originating membrane.
Abstract: Membrane scission is essential for intracellular trafficking. While BAR domain proteins such as endophilin have been reported in dynamin-independent scission of tubular membrane necks, the cutting mechanism has yet to be deciphered. Here, we combine a theoretical model, in vitro, and in vivo experiments revealing how protein scaffolds may cut tubular membranes. We demonstrate that the protein scaffold bound to the underlying tube creates a frictional barrier for lipid diffusion; tube elongation thus builds local membrane tension until the membrane undergoes scission through lysis. We call this mechanism friction-driven scission (FDS). In cells, motors pull tubes, particularly during endocytosis. Through reconstitution, we show that motors not only can pull out and extend protein-scaffolded tubes but also can cut them by FDS. FDS is generic, operating even in the absence of amphipathic helices in the BAR domain, and could in principle apply to any high-friction protein and membrane assembly.
Thème : Membranes et trafic membranaire
Equipe : Membranes et Fonctions cellulaires (LPCIC)
